 Following weeks of preparation, Saint Barnabas Medical Center (SBMC), an RWJBarnabas Health facility, began administering the Pfizer- BioNTech vaccine to its high-risk, frontline employees today. The hospital opened its employee vaccine clinic at 6:30 am on December 21, 2020, and employees were welcomed with cheers of support from the administrative, medical and volunteer staff.
Following weeks of preparation, Saint Barnabas Medical Center (SBMC), an RWJBarnabas Health facility, began administering the Pfizer- BioNTech vaccine to its high-risk, frontline employees today. The hospital opened its employee vaccine clinic at 6:30 am on December 21, 2020, and employees were welcomed with cheers of support from the administrative, medical and volunteer staff.
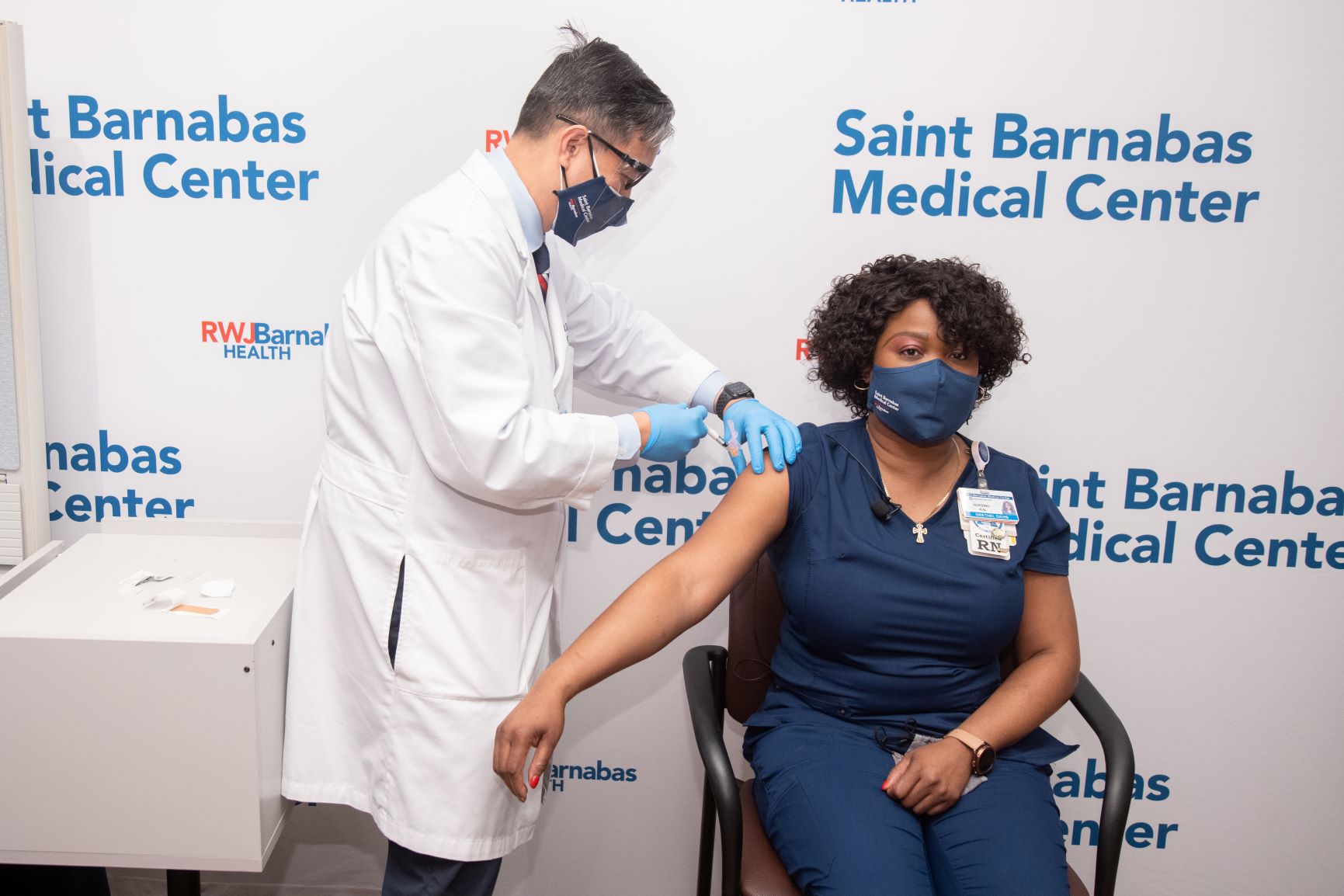
Grethel John, RN, CCRN, BSN, an Intensive Care Unit Nurse, was the first person to receive the COVID-19 vaccine at Saint Barnabas Medical Center, Livingston, New Jersey. “As an ICU nurse on the front lines, I have seen too much pain and sorrow from COVID-19. I hope I can inspire people especially those with my complexion with black and brown skin to take the vaccine and see that it is safe,” Grethel remarked.
Grethel is pictured receiving her vaccine from Ernani Sadural, MD, Director of Global Health, RWJBarnabas Health.
RWJBarnabas Health (RWJBH) has been working diligently with the state to support the New Jersey Department of Health’s ambitious vaccination plan to get 70 percent of the state’s adult population vaccinated in six months. As the largest, most comprehensive academic health care system in New Jersey, RWJBarnabas Heath is committed to treating and saving the lives of patients with COVID-19, and also to fighting the spread of the disease, protecting its team members and ending the pandemic. With the opening of its employee vaccine clinic today, Saint Barnabas Medical Center is proud to be an integral part of the national and New Jersey COVID-19 vaccination effort. Public health officials and medical experts believe vaccination is an important step in helping to prevent or lessen the effect of COVID-19 and its potentially devastating consequences.
“Since early March, we have experienced the impact of COVID-19 both locally and globally. At Saint Barnabas Medical Center, our health care heroes have cared for more than 1,500 hospitalized patients. Until now, we have been on the defense with this virus; utilizing every tool that we have to treat it. Today marks a new beginning as we start vaccinating our health care team and preventing it,” states Stephen P. Zieniewicz, FACHE, President and CEO.
The Pfizer- BioNTech vaccine received emergency use authorization by the Food and Drug Administration (FDA) on December 11, 2020. Vaccine safety and efficacy for Pfizer’s vaccine has been issued Emergency Use Authorization (EUA) by the FDA. FDA authorization of a vaccine means the agency has determined, based on substantial evidence and a stringent review process, that the vaccine is safe and effective for its intended use. The vaccine has been shown to be 95 percent effective and requires two doses received 21 days apart. The vaccine is voluntary for employees and medical staff and is being offered free of charge.
Due to limited supply, the vaccine is being given in phases based on prioritization order. The prioritization order for RWJBarnabas Health staff is determined by the risk of contracting COVID-19 from exposures while at work, primarily by job setting. RWJBarnabas Health facilities expect to vaccinate staff over a 6-week period (weeks 1-3 first dose; weeks 4-6 second dose).