
 Amisha Malhotra, MD
Amisha Malhotra, MD
Pediatric Infectious Diseases,
Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson University Hospital,
Associate Professor of Pediatrics at Rutgers-Robert Wood Johnson Medical School
COVID-19 vaccines are recommended by the Centers for Disease Control and Prevention (CDC) for children ages 6 months and older. COVID-19 vaccination protects children against severe disease, including hospitalization. Pediatric clinical trials have shown that these vaccines are safe and effective for children and ongoing safety monitoring shows that COVID-19 vaccination continues to be safe.
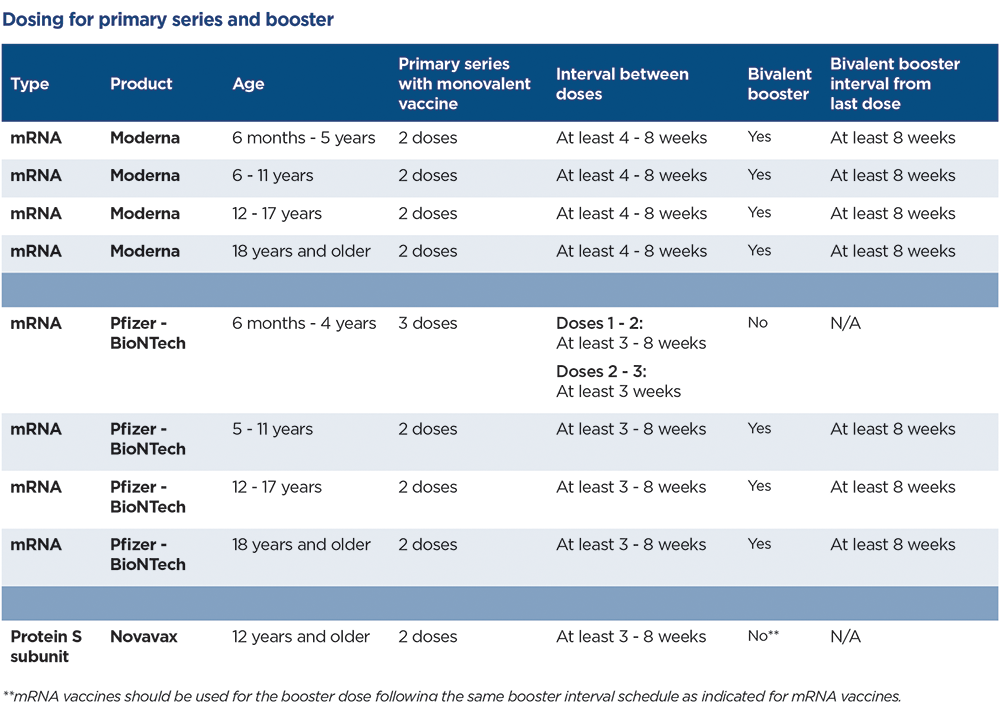
Currently there are 2 types of vaccines approved for children under the age of 18 years for primary series vaccination against COVID-19: messenger RNA (mRNA) and protein subunit vaccines. mRNA vaccines use lab-created messenger RNA as a blueprint for the body’s cells to produce immunity against the S protein of the SARs-CoV-2 virus. The protein subunit vaccines contain a lab-created portion of the S protein to help the body elicit an immune response. The mRNA vaccines, produced by Pfizer-BioNTech and Moderna are approved for children ages 6 months and older and the protein subunit vaccine, produced by Novavax, is approved for children 12 years and older. Children receive a smaller dose of COVID-19 vaccine than teens and adults. COVID-19 vaccine dosage is based on the child’s age on the day of vaccination and not on a child’s size or weight.
The primary series vaccines contain a mono or single mRNA component of the original strain of the SARS CoV-2 virus. The current booster vaccinations contain two mRNA components of the coronavirus. Half of the vaccine targets the original strain, and the other half targets the BA.4 and BA.5 Omicron subvariant lineages which are predicted to continue circulating this fall and winter. Only the mRNA vaccines are approved for booster dosing. For the best protection against COVID-19, children and adults should be ‘up-to-date’ with COVID-19 vaccination. Being ‘up-to-date is defined as having completed the primary series with the monovalent vaccine plus having received the bivalent booster.
Reported side effects of COVID vaccines are mild, temporary and similar to those experienced after routine vaccines including fever, headache, chills, muscle aches, and pain at injection site. Some children have no side effects. Children who have already had COVID-19 should still get vaccinated with COVID-19 vaccines. Emerging evidence indicates that people can get added protection by getting vaccinated after they have been infected with the virus that causes COVID-19. For children who have been infected, their next dose can be delayed 3 months from when symptoms started or, if they did not have symptoms, when they received a positive test.
In addition, children can safely receive other vaccines the same day they receive their COVID-19. Routine vaccinations, including influenza vaccine, should not be delayed. Children who are moderately or severely immunocompromised and are being vaccinated with the mRNA vaccines, should receive 3 doses of monovalent vaccine to complete the primary series. Bivalent booster dosing is recommended at least 8 weeks after completion of the 3rd dose in the primary series.
The ASP, representing pediatric providers, including pediatric infectious disease specialists and pharmacists, exchange ideas, discuss case management strategies and develop and implement guidelines to be shared system wide, as well as serve as a resource for community physicians.
For more educational information, research and best practices from the Children’s Health Network at RWJBarnabas Health, visit rwjbh.org/childrenshealthresearch.